Safer Opioid Supply Programs Evaluation in Toronto, Ontario
The CDPE is leading a study to evaluate the impact of safer opioid supply programs in Toronto, Ontario.
Project Contents
Overview
Canada continues to experience a fatal overdose epidemic resulting from the contamination of the unregulated drug supply. This has led to growing calls for piloting the provision of safer opioid supply programs (also known as safe supply), which are an extension of standard medication-assisted treatments such as methadone and buprenorphine/naloxone. In 2019, the CDPE published a commentary in the Canadian Journal of Public Health supporting the expansion of alternatives for pharmaceutical grade opioid options for people who use opioids. More recently, the CDPE participated in a scoping review on barriers to safer supply programs during COVID-19. Safer opioid supply regimens typically include an extended-release morphine sulfate and immediate release hydromorphone doses to offset or replace use of the contaminated unregulated drug supply and to thereby prevent overdose. An investment from Health Canada in September 2020 has scaled-up safer opioid supply programs.
Under the auspices of the Integrated Supervised Injection Services Evaluation, the CDPE is undertaking an evaluation of safer opioid supply programs in partnership with community healthcare agencies in downtown Toronto. This evaluation aims to uncover the impact of safer opioid supply programs by measuring health, social, and legal outcomes.
Financial Supporters
St. Michael’s Hospital Foundation
Partners
Moss Park Consumption and Treatment Service | Parkdale Queen West Community Health Centre | Regent Park Community Health Centre | South Riverdale Community Health Centre | Street Health | The Works at Toronto Public Health
Project Contact
Dr. Mohammad Karamouzian
Mohammad.Karamouzian@unityhealth.to
Related Content
Blog Posts:
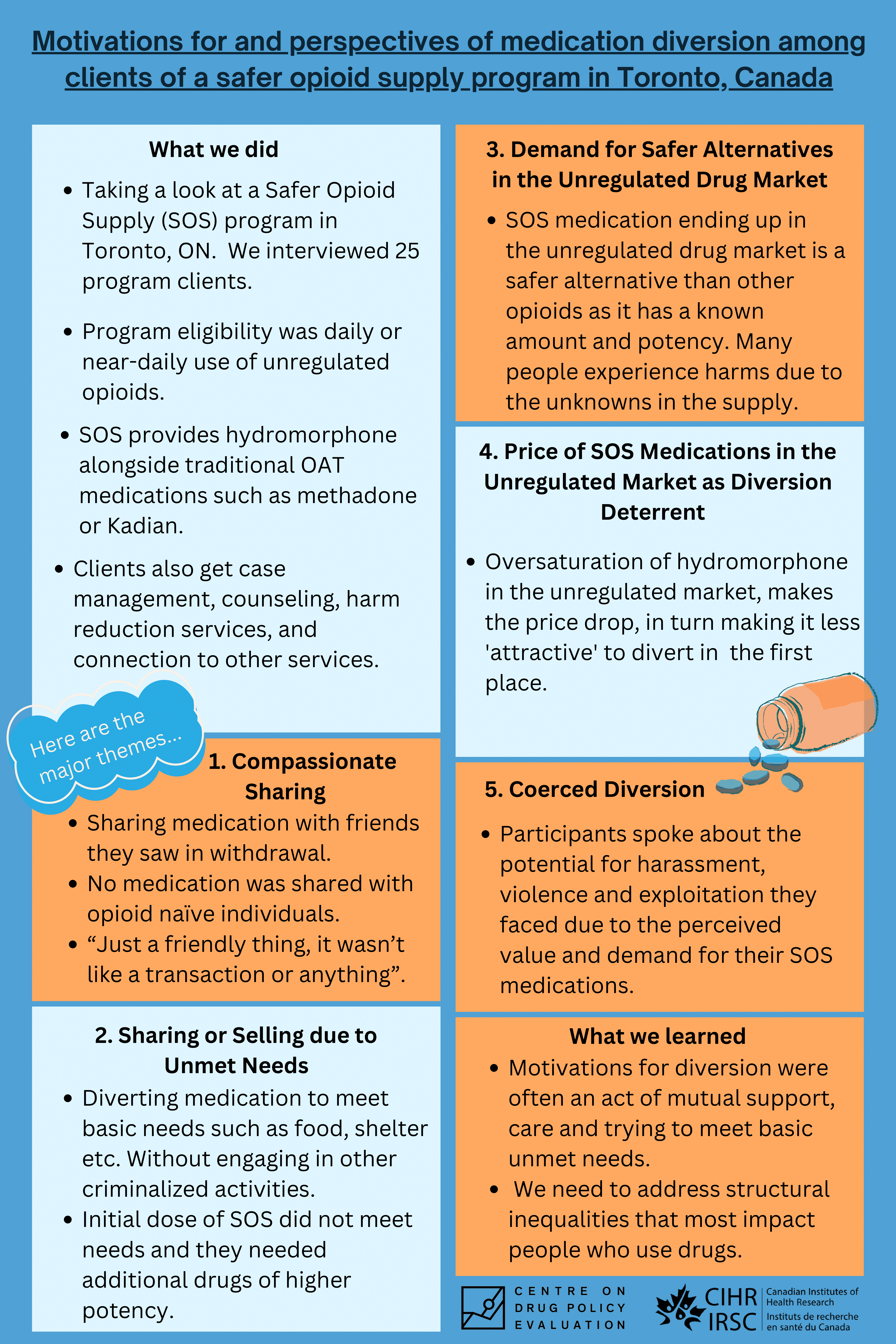
Motivations for and perspectives of medication diversion among clients of a safer opioid supply program in Toronto, Canada
Safer opioid supply programs in Canada have come under intense scrutiny related to the perceived risk of diversion of safer opioid supply medications. We sought to explore the experiences and perspectives of safer opioid supply medication diversion with clients of a safer opioid supply program in Toronto, Canada.
View
A qualitative assessment of tablet injectable opioid agonist therapy (TiOAT) in rural and smaller urban British Columbia, Canada: Motivations and initial impacts
This study explores a novel therapeutic approach combining behavioral therapy with non-opioid medication to manage chronic pain, showing improved pain relief, emotional well-being, and daily functioning in patients.
View
A Preliminary Assessment of Short-Term Social and Substance Use-Related Outcomes Among Clients of Integrated Safer Opioid Supply Pilot Programs in Toronto, Canada
This study found that clients of safer opioid supply (SOS) programs in Toronto experienced fewer overdoses and reduced use of unregulated drugs. Participants also reported improved social outcomes, suggesting SOS programs may help mitigate overdose risks.
View
Challenges of implementing safer supply programs in Canada during the COVID-19 pandemic: A qualitative analysis
This publication examines the implementation challenges faced by Canada's safer supply pilot programs, which provide pharmaceutical alternatives to individuals accessing the unregulated drug supply, highlighting organizational, structural, and procedural barriers encountered during their initial phases.
View
Access to tablet injectable opioid agonist therapy in rural and smaller urban settings in British Columbia, Canada: a qualitative study
A study in British Columbia found that rural TiOAT programs face barriers like transportation and strict dosing schedules, though participants valued the supportive, stigma-free clinic environments. Interruptions during hospital stays or incarceration highlighted gaps in continuity of care.
View
The Ontario Integrated Supervised Injection Services Cohort Study of People Who Inject Drugs in Toronto, Canada (OiSIS-Toronto): Cohort Profile
The Ontario Integrated Supervised Injection Services cohort in Toronto, Canada (OiSIS-Toronto) is an open prospective cohort of people who inject drugs established to evaluate the impacts of supervised consumption services integrated within three community health agencies on health status and service use.
View
Xylazine detected in unregulated opioids and drug administration equipment in Toronto, Canada: clinical and social implications
We report the first detection of the psychoactive veterinary compound xylazine in Toronto, the largest urban center in Canada, by the city’s drug checking service.
View
Expanding Access to Diacetylmorphine and Hydromorphone for People Who Use Opioids in Canada
This commentary explores the current state of policy and practice for diacetylmorphine and hydromorphone as opioid substitution options in Canada, outlines the rationale for rapid expansion of access, and highlights clinical and policy changes that must be undertaken or the death toll will continue to rise.
View
Securing Safe Supply During COVID-19 and Beyond: Scoping Review and Knowledge Mobilization
A scoping review was conducted in order to identify key concepts, strategies and gaps in evidence with respect to the provision of safe supply during pandemics and other large-scale emergency conditions.
View
Regulation Project
Working to create public health- and human rights-based drug policies grounded in compassion and guided by evidence.
View